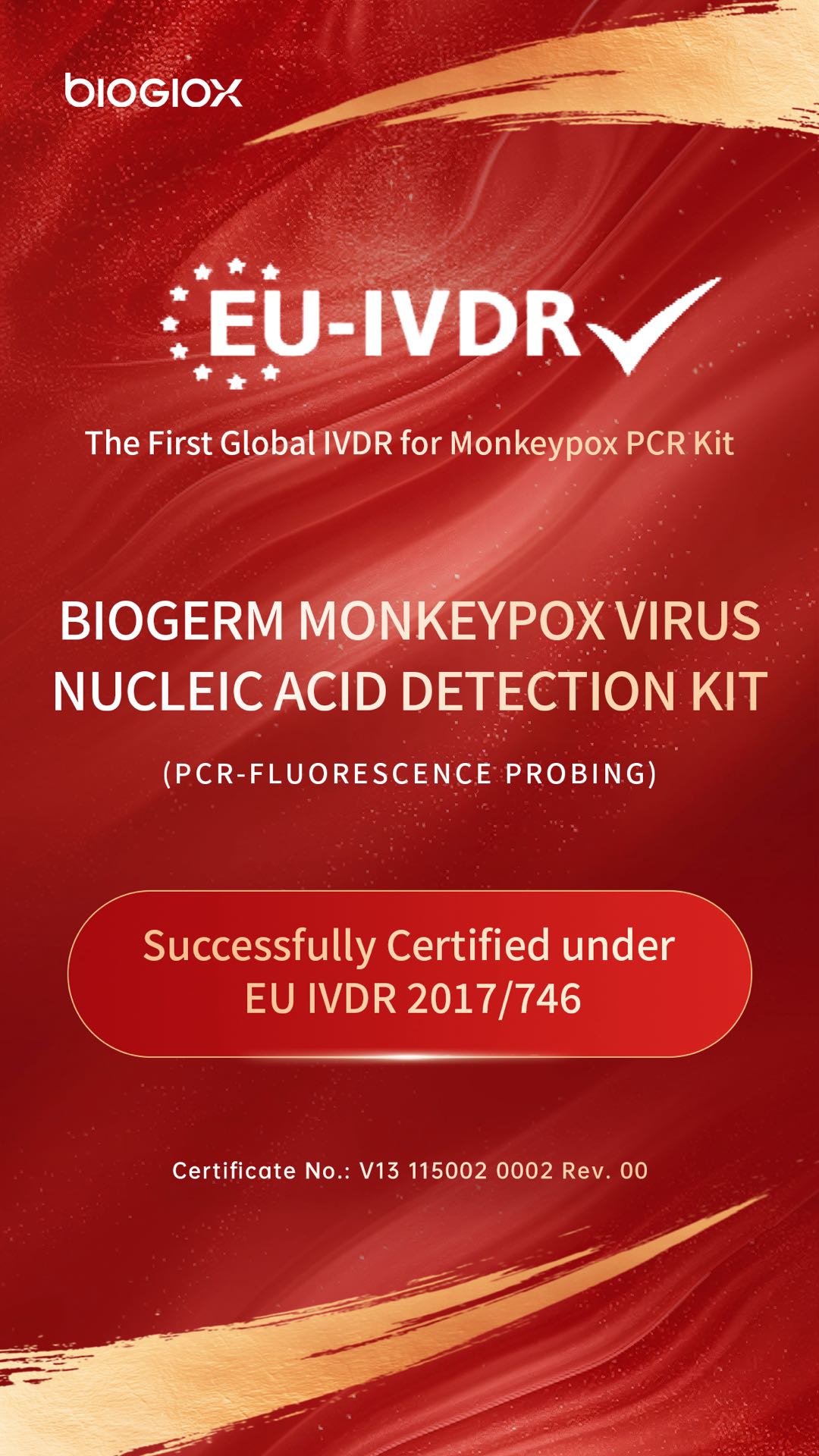
BioGioX Monkeypox Virus Nucleic Acid Detection Kit Successfully Obtains EU IVDR Certification
BioGioX Monkeypox Virus Nucleic Acid Detection Kit Successfully Obtains EU IVDR Certification
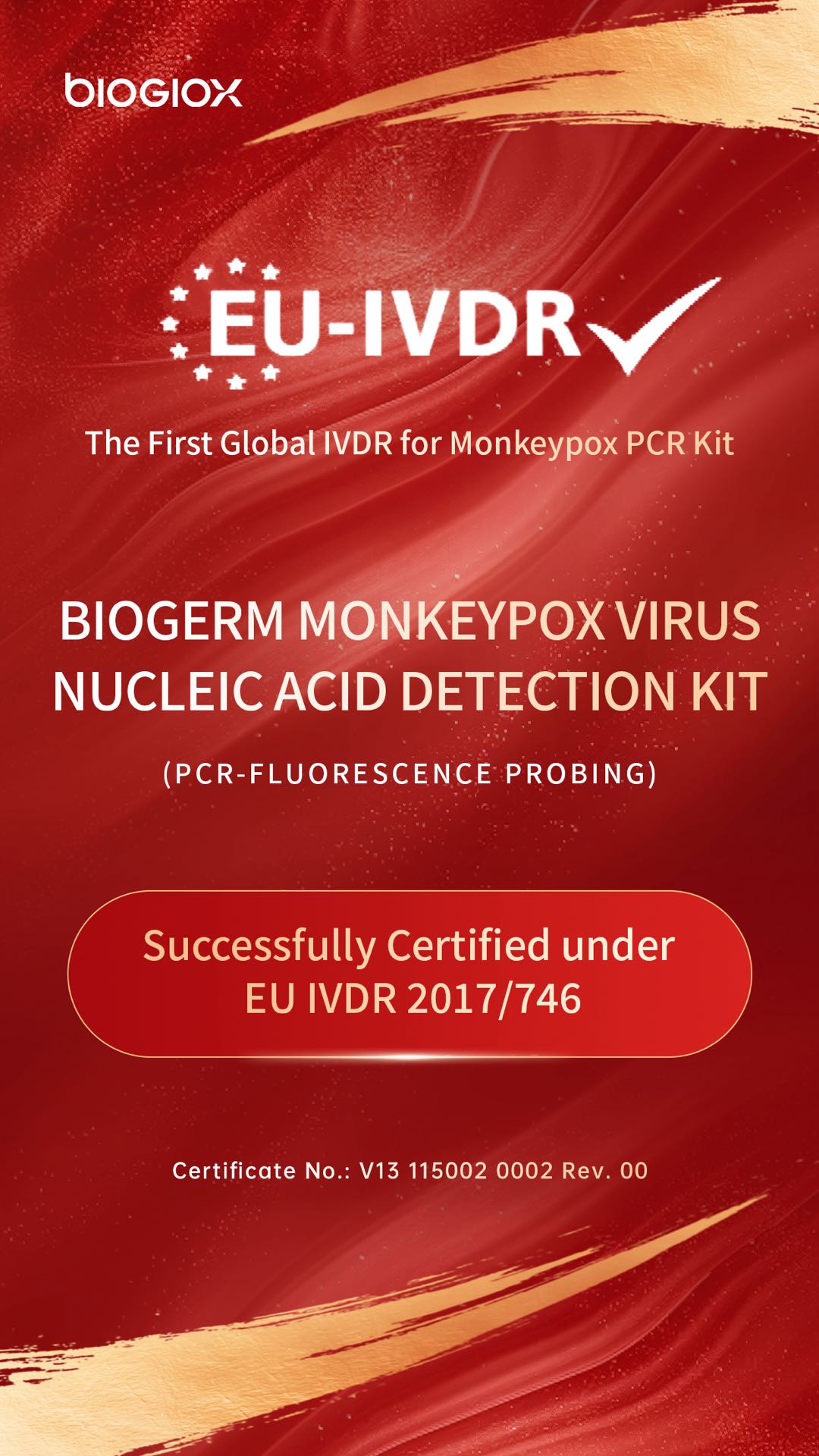
A Global First: Chinese Diagnostic Enterprise Achieves Breakthrough, Ushering in a New Era in Mpox Prevention and Control
BioGioX is proud to announce a landmark achievement: its BioGioX has successfully obtained the European Union In Vitro Diagnostic Medical Device Regulation (IVDR) certificate on September 17, 2025. This certification designates it as the first and currently only mpox nucleic acid test kit in the world to receive IVDR approval. This milestone represents a historic leap for a Chinese enterprise in the global infectious disease diagnostics sector, transitioning from following international standards to leading the way.
A Groundbreaking Achievement: Reaching the Summit of IVD Compliance
The EU IVDR certification is widely regarded as one of the most stringent regulatory standards in the in-vitro diagnostics industry. Since its enactment in 2017, the IVDR has significantly elevated regulatory requirements, posing considerable challenges for diagnostic products seeking certification.
The issuance of this global-first IVDR certificate for an mpox test (Certificate No.: V13 115002 0002 Rev. 00) to BioGermerge is not only a validation of the product's exceptional performance but also powerful testament to the company's world-class capabilities in R&D, quality management, and clinical validation.
This exclusive breakthrough establishes BioGermerge's significant technological leadership in mpox diagnostics, providing a testing solution for global mpox prevention and control that meets the IVDR's high standards.
Global Mpox Situation and Diagnostic Challenges
In a recent mpox situation report, the World Health Organization (WHO) noted that while the global mpox outbreak has generally stabilized, new virus variants and transmission patterns continue to emerge, demanding higher diagnostic accuracy. In pathogen detection, fluorescent PCR technology is recognized as the "gold standard" for laboratory confirmation due to its high sensitivity and specificity. Compared to methods like colloidal gold, primarily used for initial screening, PCR enables more accurate virus identification in the early stages of infection, significantly reducing the risk of false negatives.
The rapid spread of the new Clade I 'subtype' variant, from the Democratic Republic of the Congo to several East African nations, further underscores the critical need for precise diagnostics. In this context, the launch of BioGermerge's uniquely IVDR-certified product is a timely development, offering a reliable solution for global public health security.
Recent reports from the Hong Kong Department of Health indicate ongoing local transmission, with 12 confirmed cases recorded this year to date. Accurate and rapid diagnostic tools are crucial for breaking transmission chains.
Global Impact: Reshaping the Mpox Diagnostics Landscape
BioGermerge's exclusive IVDR certification is set to redefine the competitive landscape of the mpox diagnostics market.
This breakthrough signifies that:
Healthcare institutions in EU member states are mandated to prioritize the procurement of IVDR-compliant diagnostic products.
A gold-standard testing tool is now available for global mpox outbreak control.
A Chinese diagnostics enterprise has once again secured a voice in setting international standards within the infectious disease testing arena.
"This is more than just a certificate; it is the highest recognition from global regulators and the healthcare market for BioGermerge's technological prowess," stated a company representative.
'Made-in-China' Innovation Safeguarding Global Public Health
Securing this global-first IVDR certification is just the beginning. BioGermerge is accelerating registration and application processes in more countries, aiming to make this internationally top-tier testing product accessible to healthcare institutions worldwide.
As mpox virus variants continue to emerge and its spread potentially widens, diagnostic tools conforming to the highest quality standards will be central to outbreak control. With its uniquely IVDR-certified product, BioGermerge has not only earned acclaim for the Chinese diagnostics industry but has also contributed Chinese expertise to building a global community for public health.
For further information, please contact:
Overseas Sales: Ms. Li +86-18665998757
Business Development: Mr. Meng +86-18515455125